Hypotheses about the origins of life may be divided into several categories. Most approaches investigate how self-replicating molecules or their components came into existence.
For example, the Miller–Urey experiment and similar experiments demonstrated that most amino acids, often called "the building blocks of life", were shown to be racemically synthesized in conditions similar to those of the early Earth. Several mechanisms have been investigated, including lightning and radiation. Other approaches ("metabolism first" hypotheses) focus on understanding how catalysis in chemical systems in the early Earth might have provided the precursor molecules necessary for self-replication.
When did life originate?
Evidence suggests that life first evolved around 3.5 billion years ago. This evidence takes the form of microfossils (fossils too small to be seen without the aid of a microscope) and ancient rock structures in South Africa and Australia called stromatolites. Stromatolites are produced by microbes (mainly photo synthesizing cyanobacteria) that form thin microbial films which trap mud; over time, layers of these mud/microbe mats can build up into a layered rock structure — the stromatolite.
Stromatolites are still produced by microbes today. These modern stromatolites are remarkably similar to the ancient stromatolites which provide evidence of some of the earliest life on Earth. Modern and ancient stromatolites have similar shapes and, when seen in cross section, both show the same fine layering produced by thin bacterial sheets. Microfossils of ancient cyanobacteria can sometimes be identified within these layers.
Where / How did life originate?
Scientists are exploring several possible locations for the origin of life, including tide pools and hot springs. There 7 most famous theory about how this life originated.
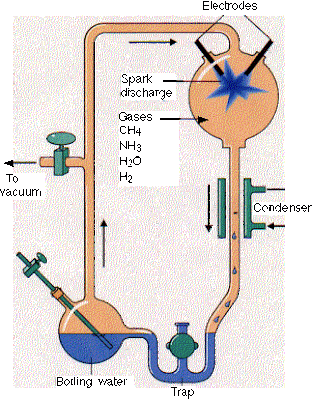
Electric Spark - Miller's Experiment
Electric sparks can generate amino acids and sugars from an atmosphere loaded with water, methane, ammonia and hydrogen, as was shown in the famous Miller-Urey experiment reported in 1953, suggesting that lightning might have helped create the key building blocks of life on Earth in its early days.
Over millions of years, larger and more complex molecules could form. Although research since then has revealed the early atmosphere of Earth was actually hydrogen-poor, scientists have suggested that volcanic clouds in the early atmosphere might have held methane, ammonia and hydrogen and been filled with lightning as well.
Community Clay
The first molecules of life might have met on clay, according to an idea elaborated by organic chemist Alexander Graham Cairns-Smith at the University of Glasgow in Scotland. These surfaces might not only have concentrated these organic compounds together, but also helped organize them into patterns much like our genes do now.
The main role of DNA is to store information on how other molecules should be arranged. Genetic sequences in DNA are essentially instructions on how amino acids should be arranged in proteins. Cairns-Smith suggests that mineral crystals in clay could have arranged organic molecules into organized patterns. After a while, organic molecules took over this job and organized themselves.
Recently some scientists have narrowed in on the hypothesis that life originated near a deep sea hydrothermal vent. The chemicals found in these vents and the energy they provide could have fueled many of the chemical reactions necessary for the evolution of life.
Furthermore, using the DNA sequences of modern organisms, biologists have tentatively traced the most recent common ancestor of all life to an aquatic microorganism that lived in extremely high temperatures — a likely candidate for a hydrothermal vent inhabitant!
Chilly Start
Ice might have covered the oceans 3 billion years ago, as the sun was about a third less luminous than it is now. This layer of ice, possibly hundreds of feet thick, might have protected fragile organic compounds in the water below from ultraviolet light and destruction from cosmic impacts. The cold might have also helped these molecules to survive longer, allowing key reactions to happen.
Nowadays DNA needs proteins in order to form, and proteins require DNA to form, so how could these have formed without each other? The answer may be RNA, which can store information like DNA, serve as an enzyme like proteins, and help create both DNA and proteins.
Later DNA and proteins succeeded this "RNA world," because they are more efficient. RNA still exists and performs several functions in organisms, including acting as an on-off switch for some genes. The question still remains how RNA got here in the first place. And while some scientists think the molecule could have spontaneously arisen on Earth, others say that was very unlikely to have happened.
Other nucleic acids other than RNA have been suggested as well, such as the more esoteric PNA or TNA.
Instead of developing from complex molecules such as RNA, life might have begun with smaller molecules interacting with each other in cycles of reactions. These might have been contained in simple capsules akin to cell membranes, and over time more complex molecules that performed these reactions better than the smaller ones could have evolved, scenarios dubbed "metabolism-first" models, as opposed to the "gene-first" model of the "RNA world" hypothesis.
Panspermia
Perhaps life did not begin on Earth at all, but was brought here from elsewhere in space, a notion known aspanspermia. For instance, rocks regularly get blasted off Mars by cosmic impacts, and a number of Martian meteorites have been found on Earth that some researchers have controversially suggested brought microbes over here, potentially making us all Martians originally.
This meteorite, that fell near Murchison, Australia on 28 September 1969, turned out to contain a variety of organic molecules including:
- purines and pyrimidines
- polyols — compounds with hydroxyl groups on a backbone of 3 to 6 carbons such as glycerol and glyceric acid. Sugars are polyols.
- the amino acids listed in this table. The amino acids and their relative proportions were quite similar to the products formed in Miller's experiments.
The question is: were these molecules simply terrestrial contaminants that got into the meteorite after it fell to earth?
Probably not:
- Some of the samples were collected on the same day it fell and subsequently handled with great care to avoid contamination.
- The polyols contained the isotopes carbon-13 and hydrogen-2 (deuterium) in greater amounts than found here on earth.
- The samples lacked certain amino acids that are found in all earthly proteins.
- Only L amino acids occur in earthly proteins, but the amino acids in the meteorite contain both D and L forms (although L forms were slightly more prevalent).

Other scientists have even suggested that life might have hitch hiked on comets from other star systems. However, even if this concept were true, the question of how life began on Earth would then only change to how life began elsewhere in space.
Source :
**************************************
Melaka Planetarium Adventure Science Centre










0 comments:
Post a Comment